

This is recapped in the video and the balanced symbol equations are introduced. Learners may know the products of a metal + acid reaction and be able to write a word equation. Learners may have experience of investigating the relative reactivity of some metals at 11–14 using a simplified method, such as counting bubbles in a test tube. They should know the definition of an element and be able to identify metals and non-metals using the periodic table.
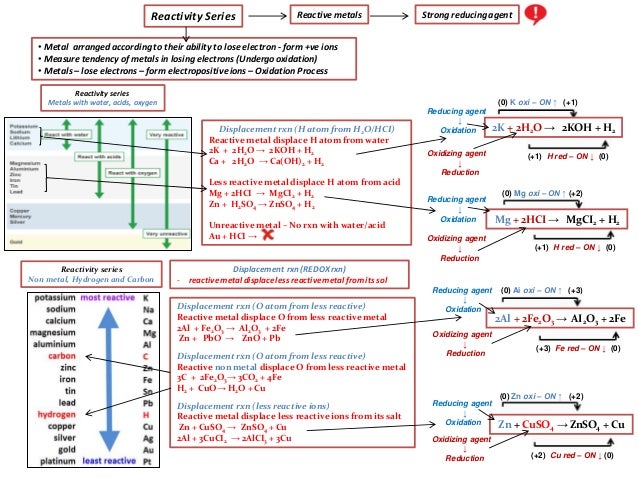
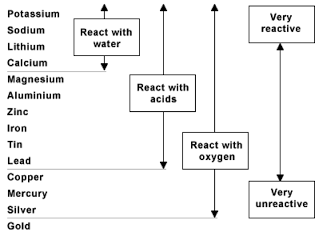
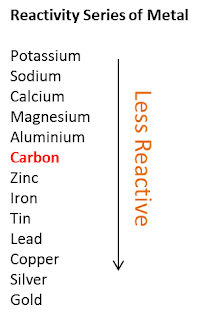
Learners should be familiar with the periodic table of elements. Learners will need to have a clear understanding of the following scientific terminology: Tin will be more reactive than copper but less reactive than iron. Learners could repeat the displacement reactions experiment and include the unknown metal and metal sulfate. To add additional higher level extension to this content, you could introduce an unknown metal and its metal salt and then ask the pupils to do an experiment to find out where it fits in the reactivity series, for example you could use tin and tin(II) sulfate.
Extension work based on the reactivity of metals practical Printable results tables are provided in the supporting resources booklet to save time in your practical lessons.
#Metal ion reactivity series how to
This section of the video also looks at how to do a fair test. Caution learners against using the thermometer to agitate the metal in the acid as there is a risk of making a hole in the cup as well as damaging the thermometer. In the metal acid reactions observe the recommended concentration for the acid as there will not be a significant temperature rise at lower concentrations. TIP You may need to remove the metal in the copper(II) sulfate solution to see that it has a brown layer of solid on it – it will look black when in the blue solution. Consider demonstrating the copper(II) sulfate reactions on a larger scale, so learners can clearly see a change in the colour of solution – it may be harder to spot in the dimple tray.
This minimises the amount of chemicals used. The displacement reactions are carried out in microscale using a spotting tile. These investigations may be spread over two or three lessons to make the content manageable. Exothermic reactions of metals and acid and metal displacement reactions are experiments that learners can carry out themselves. The first explores the reactions of the alkali metals in water and is a teacher demonstration. The video offers three experiments that investigate the relative reactivity of metals. Notes on running the practical experiments
#Metal ion reactivity series plus
Plus technician notes and integrated instructions. Editable versions of all worksheets and key documents are provided.įull teacher notes are available in the supporting resources booklet, including ideas for how to use this video and the supporting resources as part of your teaching. Supporting resources booklet including pause-and-think questions with answers, teacher notes, intended outcomes, follow-up worksheets and structure strips.


 0 kommentar(er)
0 kommentar(er)
