
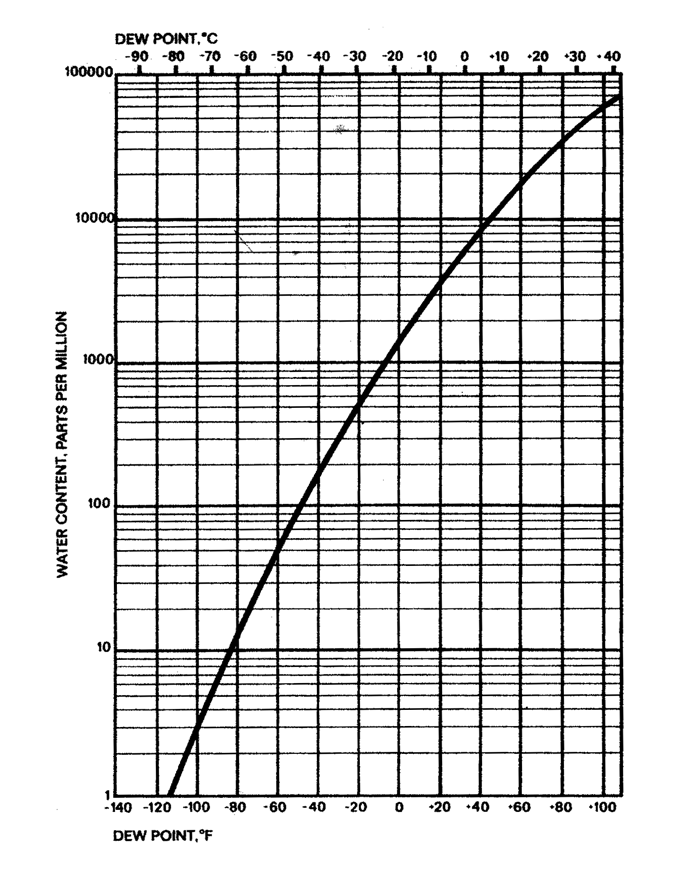
Is the relative humidity of the gas mixture being considered Relative humidity is expressed as a percentage and is calculated as follows: If the liquid is open to the air, then the vapor pressure is seen as a partial pressure along with the other constituents of the air.įor the sake of completeness, “Relative Humidity” is defined as the ratio of the partial pressure of water vapor in a gaseous mixture of air and water vapor, to the saturated vapor pressure of water-at a given temperature. Since the molecular kinetic energy is greater at higher temperature, more molecules can escape the surface and the saturated vapor pressure is correspondingly higher. At this point the vapor is said to be saturated, and the pressure of that vapor (usually expressed in millimeters of mercury ) is called the saturated vapor pressure. Evaporation will proceed until there are as many molecules returning to the liquid as there are escaping. The notion of “Saturated Vapor Pressure” can be understood by considering the process of evaporation in a closed container of water, with some air above the liquid. If the air is cooled further, some of the moisture will condense. “Dew Point” is defined as the temperature to which a given volume of air must be cooled at constant pressure and constant water vapor content in order for saturation to occur. Service Videos-for your Interscan products.Operating Instruction Videos-for your Interscan products.Installation Videos-for your Interscan products.Manuals, schematics, firmware, and software.
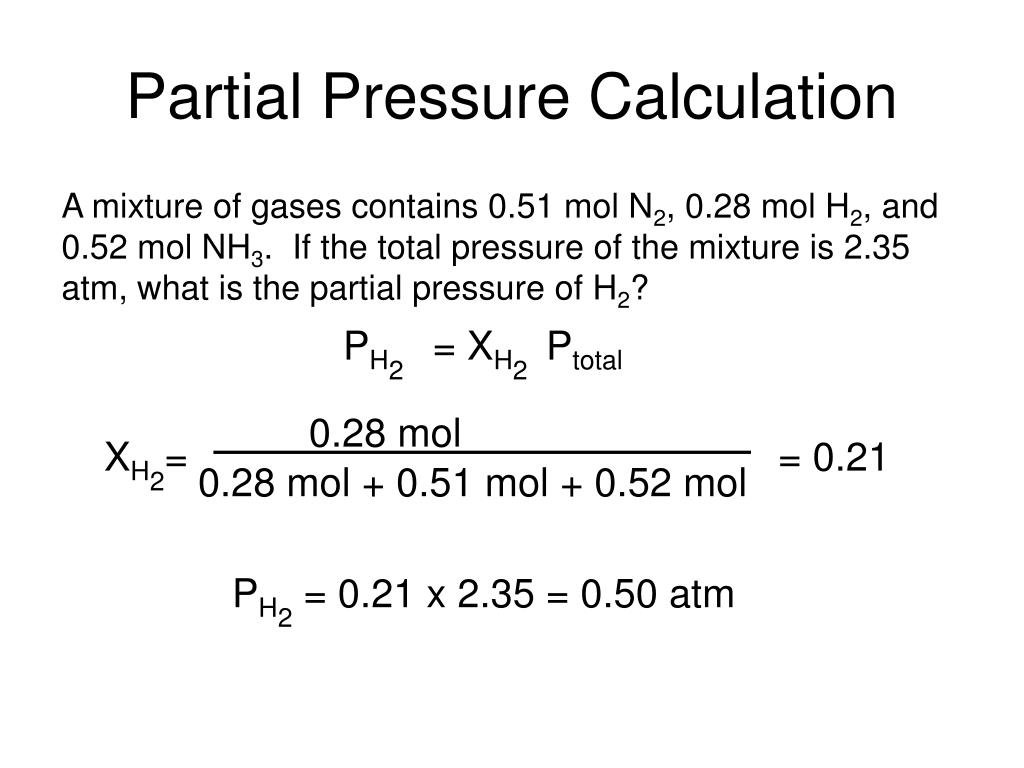
Partial pressure of water from dew point calculator portable#
GasD ® 8000 Series – Portable / Survey Gas Monitoring Applications.AccuSafe – Continuous Gas Monitoring (Single Point or Multi-Point).Your Gas Detection Headquarters on the Web


 0 kommentar(er)
0 kommentar(er)
